Abstract
Introduction Asparaginase-containing, pediatric-inspired regimens (PIR) have excellent outcomes in pediatric patients (≤ 14 years) with ALL. Clinical guidelines also recommend PIR to treat AYA patients (15-39 years) with ALL, a patient population with poorer survival relative to pediatric patients. However, the use of PIR in AYA patients with ALL is inconsistent, with some adult centers still favoring non-PIR adult regimens. Comparative analyses of survival outcomes among AYA patients with ALL receiving PIR vs. non-PIR regimens are lacking. In this retrospective study, we compared overall survival (OS), rates of allogeneic stem-cell transplant (SCT), and adverse event (AE) rates among AYA patients with newly-diagnosed ALL treated with PIR vs. non-PIR.
Methods This is a retrospective study using Optum's de-identified Clinformatics® Data Mart (Jan 2007 - Sep. 2020), which is derived from a database of administrative health claims for members of large US commercial and Medicare Advantage health plans. AYA patients with newly-diagnosed ALL between July 1, 2007 through Sep 30, 2020 who initiated ALL treatment within 6 months were included. The earlier of the date of ALL diagnosis or start of ALL treatment was designated as the index date. Patients with <6 months' continuous health plan enrollment or records of ALL remission, relapse, SCT or other cancer types in the time period before the index date (baseline period) were excluded. Patients were followed until the earlier of death, health plan disenrollment or end of study period. Patient characteristics and Charlson Comorbidity index (CCI) in the baseline period were compared between PIR vs. non-PIR patients using t-tests or Chi-squared tests. Propensity score matching was conducted to balance the baseline characteristics between PIR and non-PIR patients. OS was compared in matched PIR vs. non-PIR patients using Kaplan-Meier (KM) method and univariate Cox proportional hazards regression model in the overall population and by pre-defined subgroups. Two-year rates of SCT and common AEs (any grade) occurring in the one-year follow-up period were reported among matched groups.
Results A total of 599 AYA patients with ALL met the selection criteria, of which 187 (30%) received PIR and 303 (51%) received non-PIR. The remaining 109 (18%) patients were excluded from primary analyses due to insufficient treatment information. Propensity score matching resulted in 187 patients per group with balanced baseline characteristics. In the PIR and non-PIR patients, respectively, mean ages were 21.3 and 22.8 years; 75.4% and 66.3% were males; 12.3% and 10.2% were Hispanic; and 17.6% and 19.8% had a diagnosis of overweight/obesity at baseline. The CCI score was 0 in both groups. Mean (SD) follow-up duration was 3.1 (2.9) and 3.0 (3.0) years, respectively.
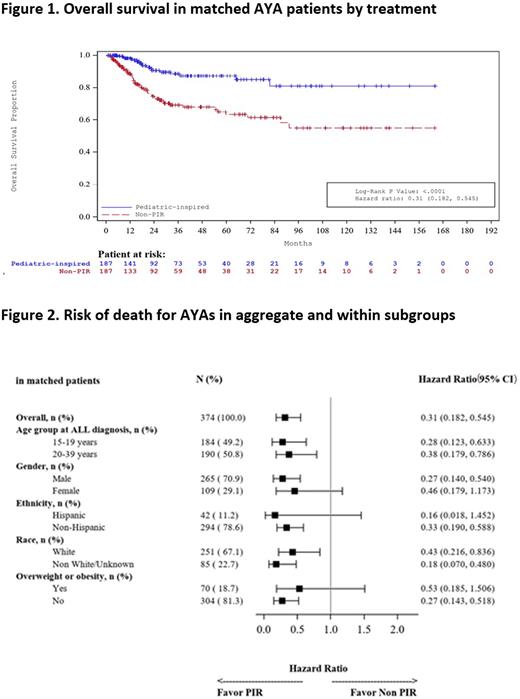
In aggregate, AYAs who received PIR had significantly higher OS (Figure 1; log rank p<0.01) with 69% lower likelihood of death as compared to AYAs who received non-PIR (hazard ratio [95% CI]: 0.31 [0.18, 0.55]). The 5-year OS estimate (95% CI) was 87.3% (81.0, 91.6) among PIR patients and 63.3% (55.2, 70.3) among non-PIR patients. The 1-year OS estimates were 98.1% (95.2, 99.2) and 88.3% (83.5, 91.7), respectively. The OS benefit for PIR was retained within subgroups (Figure 2). A significantly lower proportion of AYAs treated with PIR underwent SCT compared to non-PIR (2-year rates: 16.5% vs 33.4%; log rank p<0.01). For the PIR and non-PIR patients, respectively, common AEs within the 1-year follow-up period were: infection (61% vs. 76%), hyperglycemia (31% vs. 22%), hypokalemia (30% vs. 40%), hyperuricemia (26% vs. 25%), mucositis (25% vs. 16%) and thrombosis (9% vs. 20%). Results consistent with the primary analysis were obtained in multiple sensitivity analyses using conservative regimen definitions and varied time periods.
Conclusions AYA patients with ALL treated with a PIR had significantly better OS as compared to those treated with a non-PIR regimen, in aggregate and within pre-defined subgroups. These results suggest that PIRs may represent a superior treatment approach for most AYAs with newly-diagnosed ALL. Lower usage of SCT by PIR AYAs may have important implications for long-term health. Additional studies are needed to determine potential impacts of ALL subtypes and other clinical, hospital, and supportive care characteristics to further understand the impact of PIR on AYA survival and survivorship.
Disclosures
Bhagnani:Servier Pharmaceuticals: Current Employment. Zhu:Servier Pharmaceuticals: Current Employment. O'Dwyer:BEAM Therapeutics: Consultancy. Meng:Servier Pharmaceuticals: Current Employment. Tang:Servier Pharmaceuticals: Current Employment. Paglia:Servier Pharmaceuticals: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal